Pfizer and Cortrium: This is how a successful sales partnership works

An innovative long-term ECG on the one hand, an established sales structure on the other – this is the initial situation of the sales partnership between Pfizer and Cortrium. The Pfizer sales force has been selling the long-term ECG of the Danish medical technology startup on the German market for five years now. A best-practice example that shows what a successful sales partnership between a medical technology startup and an established pharmaceutical company can look like.
Challenges for MedTech-Startups
Developing medical technology is a challenge in view of the complex laws and rules in Germany. But not only development, CE certification, but also sales are lengthy and costly. After development and certification, startups like Cortrium face the challenge of sales: building up a large sales force is personnel, cost and time intensive and is therefore rarely practiced in reality. One solution is to cooperate with a company that has already built up its customer relationships and contacts over years and thus has a well-functioning sales structure – like Pfizer.
Pfizer and Cortrium: pioneers in sales partnership
Cortrium was founded in November 2014 by Jacob Eric Nielsen and Dr. Erik S. Poulsen in Copenhagen. The two founders built their company with a team of skilled engineers and then looked for country-based sales partners to help them market their smart long-term ECG solution in the EMEA region and other international markets. The Pfizer Healthcare Hub Berlin brought the two players together, and in 2017 Cortrium signed a sales partnership agreement with Pfizer Pharma GmbH, the first contract of its kind in Germany. In early 2018, Cortrium received the CE mark and the ISO 13485 certification as a manufacturer of long-term ECG devices. In September 2018, Cortrium launched the C3 Holter monitor on the German market.
What’s in it for Pfizer?
Atrial fibrillation is the most common cardiac arrhythmia in the general population: there are currently about 10 million patients with atrial fibrillation in Europe and between 100,000 and 200,000 new cases are added each year. Atrial fibrillation is the leading cause of strokes, heart attacks and mortality. However, early diagnosis and timely treatment can reduce this risk – this is the goal of Pfizer's activities in the area of "Screening and Diagnosis of Atrial Fibrillation". To this end, Pfizer is pursuing various projects ranging from disease awareness to the diagnostic area.
"There is currently no comparable product on the German market for the detection of cardiac arrhythmias – we are all the more pleased to be able to offer our customers such an innovative and user-friendly long-term ECG. The distribution of the C3⁺ Holter Monitor brings us a good deal closer to our goals in the area of "screening and diagnosis of atrial fibrillation"," said Bertram Weber, Manager Healthcare Strategies at Pfizer.
The product: The C3⁺ Holter Monitor
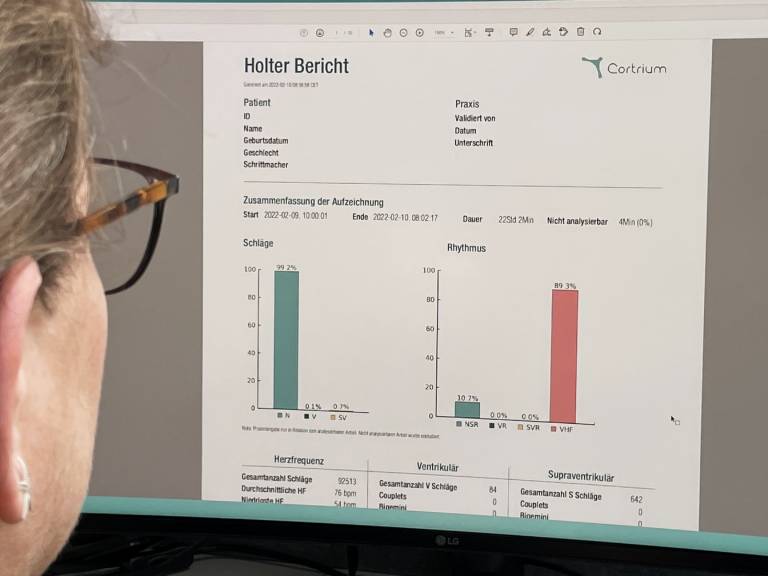
The C3⁺ Holter monitor is a wireless long-term ECG for the detection of cardiac arrhythmias, which can be easily and quickly attached to the patient's chest with standard electrodes. Due to its light weight, the device is hardly noticeable when worn. The C3⁺ records high-quality ECGs on three channels, which are analyzed using the Cortrium Analysis System.
The evaluation is algorithm-based and is backed up by human quality control. The algorithms are CE-certified and guarantee a sensitivity of 94% for the detection of ventricular beats and 95% for the detection of episodes of atrial fibrillation. Physicians receive an ECG report after 24 hours at the latest, on the basis of which they can make the diagnosis.
Sales partnership with Pfizer: What's in it for Startups and MedTech companies?
Immediately after signing the contract, Cortrium had access to Pfizer's sales structure and established customer relationships: Initially, the C3⁺ Holter Monitor was distributed by a team of trained medical device consultants. They positioned the C3⁺ Holter Monitor with interested physicians, and sales were later expanded: In addition to this specialized sales force, cardiovascular sales representatives now also promote the innovative long-term ECG. For more detailed information about the C3⁺ Holter monitor, they refer to the specially trained medical product consultants.
Product responsibility remains with Cortrium
Responsibilities are clearly defined between Cortrium and Pfizer: Sales, delivery, customer support and, of course, product responsibility is and remains with Cortrium. Nevertheless, close cooperation between the two partners is essential. Cortrium produces marketing materials for the sales force and informs the sales managers about product innovations, while Pfizer employees pass on their experience and feedback from physicians to Cortrium's product developers and customer support team, and joint solutions are developed as needed.
Cocreation at its best
While Pfizer's sales force is concentrating on expanding the sales structure and achieving the defined sales targets, Cortrium is focusing on the further development of the product: the algorithm-based Cortrium analysis system was presented at the end of 2021. Both Cortrium and Pfizer agree that the cooperation is a win-win for both sides, from which physicians, healthcare professionals – but above all patients – also benefit.
Sources:
(1) Bouwe P. Krijthe, Anton Kunst, Emelia J. Benjamin, Gregory Y.H. Lip, Oscar H. Franco, Albert Hofman, Jacqueline C.M. Witteman, Bruno H. Stricker, Jan Heeringa; Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060, European Heart Journal, Volume 34, Issue 35, 14 September 2013, Pages 2746–2751
(2) Camm AJ et al.: E.S.C. Committee for Practice Guidelines. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33(21): 2719–47.
(3) Kirchhof, P., Benussi, S., Kotecha, D., Ahlsson, A., Atar, D., Casadei, B., … & Hindricks, G. (2016). 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European heart journal, 37(38), 2893-2962.
(4) Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213-20.